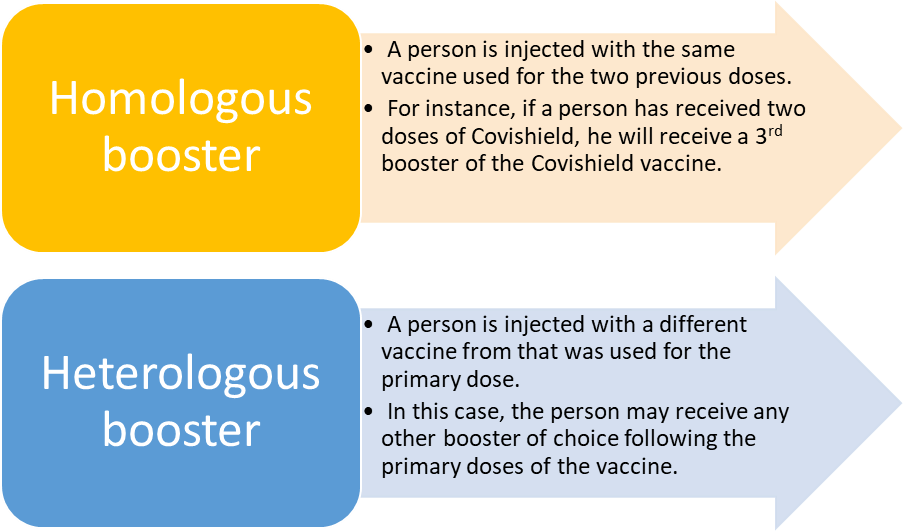
Vaccines | Free Full-Text | Heterologous Booster Dose with CORBEVAX following Primary Vaccination with COVISHIELD Enhances Protection against SARS-CoV-2

Heterologous booster response after inactivated virus BBIBP-CorV vaccination in older people - The Lancet Infectious Diseases
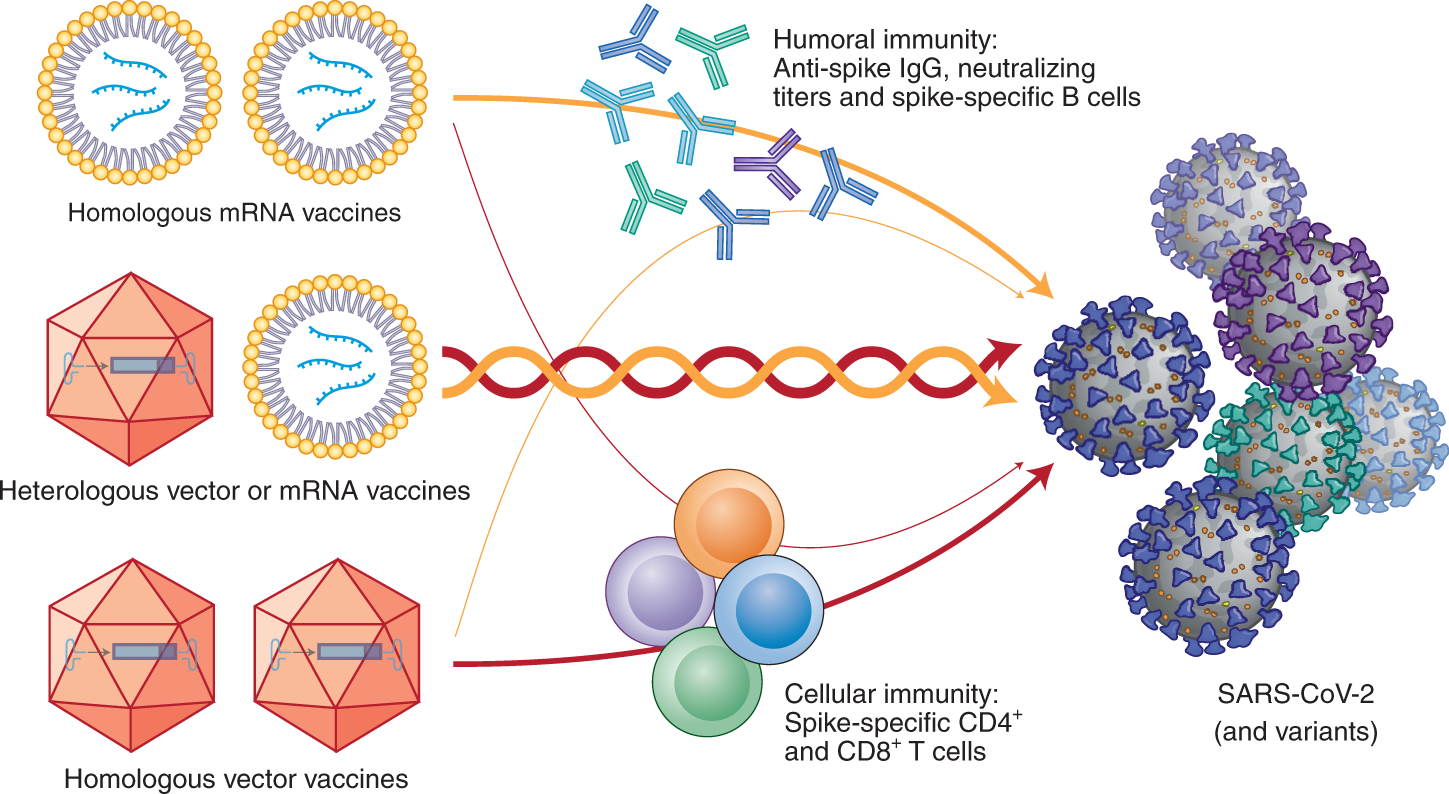
Study shows enhanced immunogenicity of heterologous boosting strategy in individuals primed with J&J vaccine

Comparative effectiveness of heterologous third dose vaccine schedules against severe covid-19 during omicron predominance in Nordic countries: population based cohort analyses | The BMJ
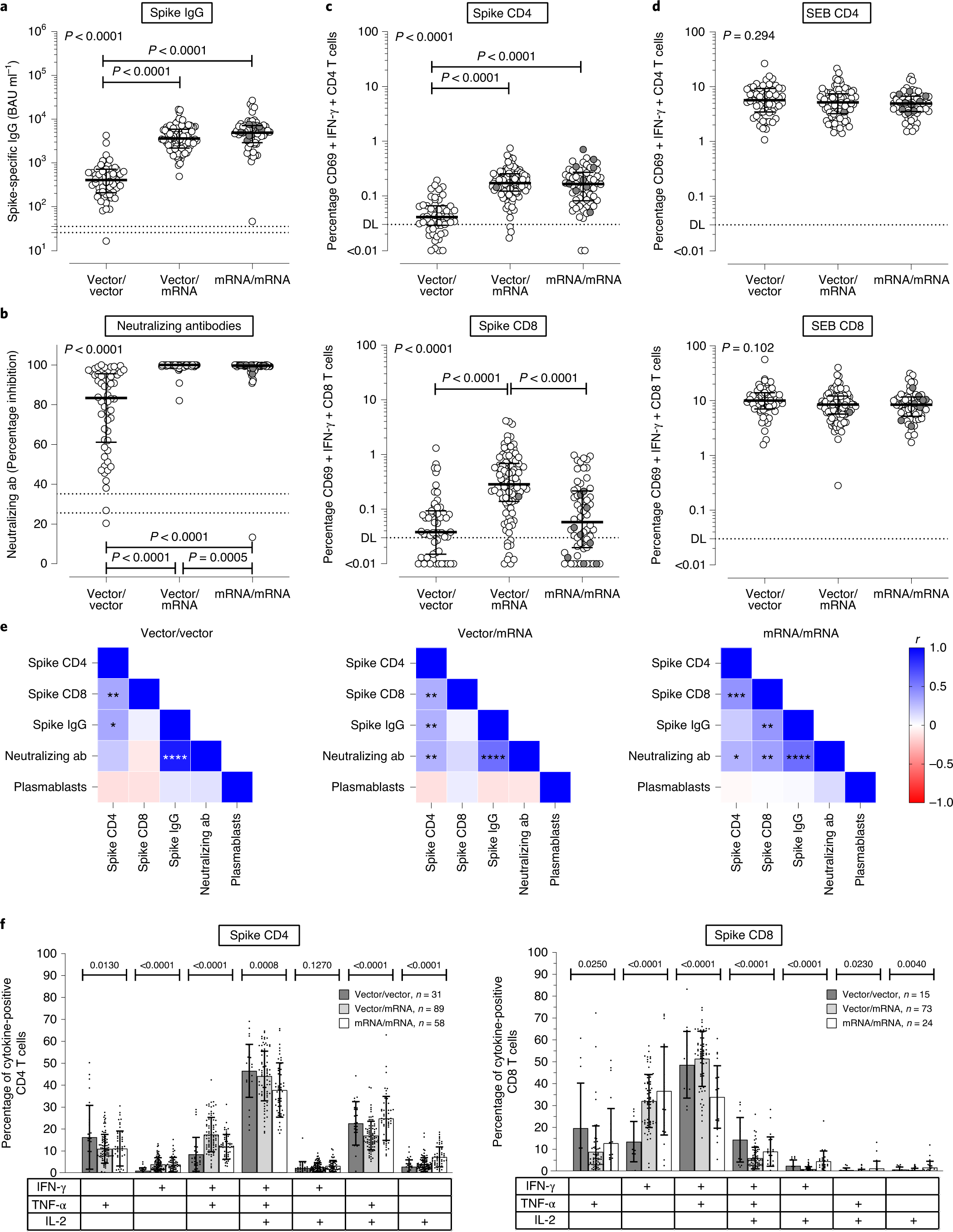
Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination | Nature Medicine

Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial - The Lancet

Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine - ScienceDirect

Safety, immunogenicity, and efficacy of the mRNA vaccine CS-2034 as a heterologous booster versus homologous booster with BBIBP-CorV in adults aged ≥18 years: a randomised, double-blind, phase 2b trial - The Lancet
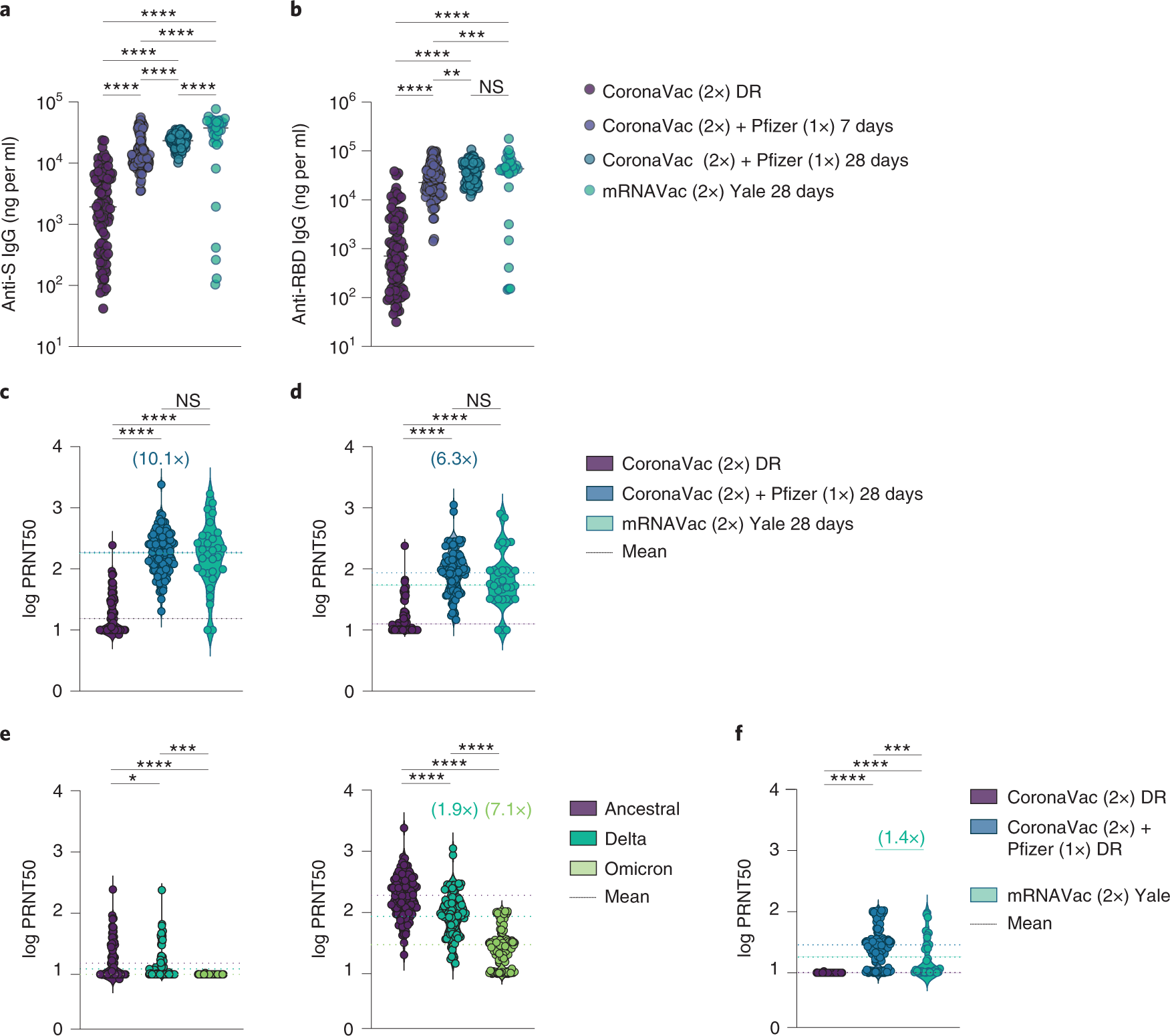
Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination | Nature Medicine
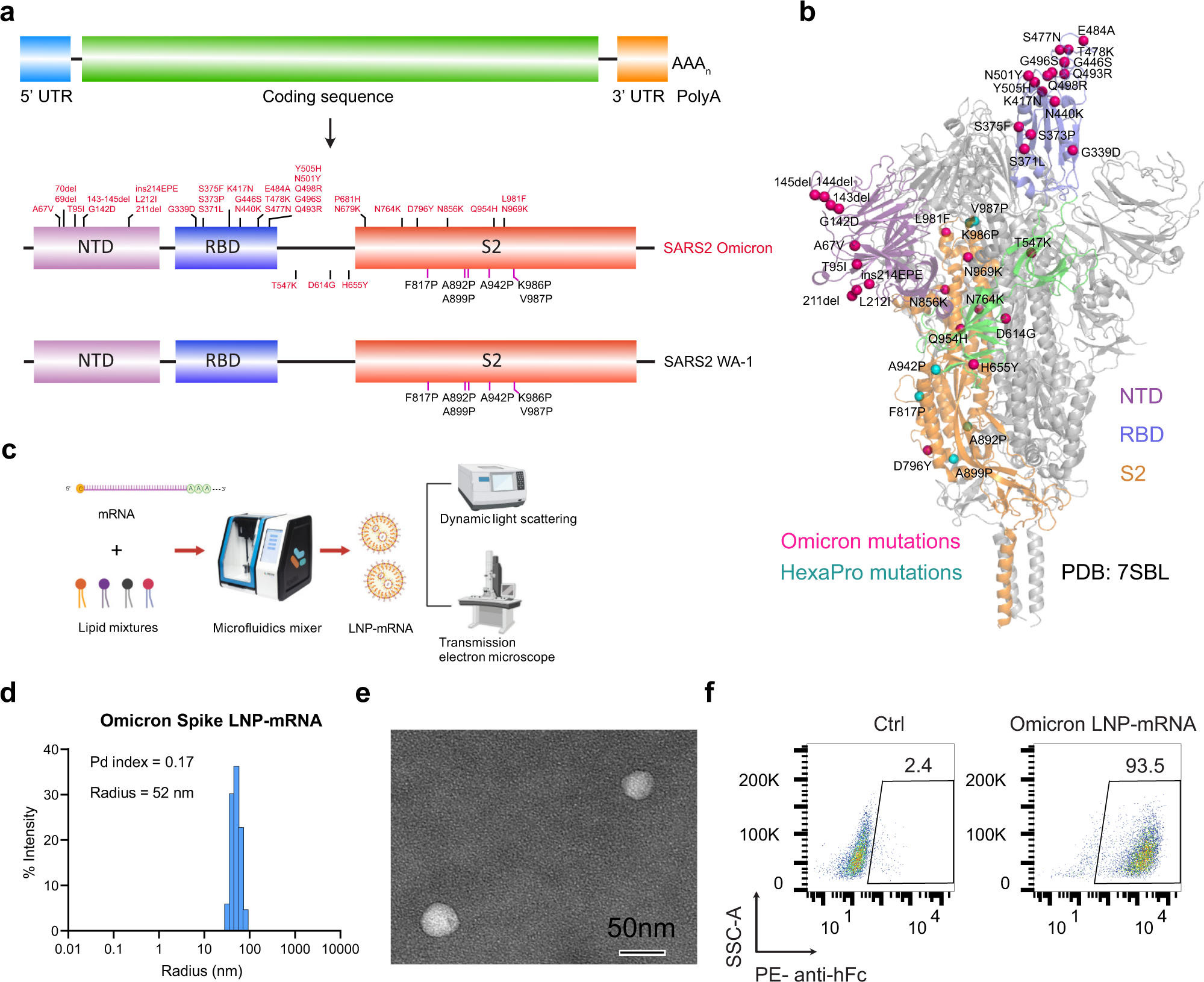
Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2 | Nature Communications
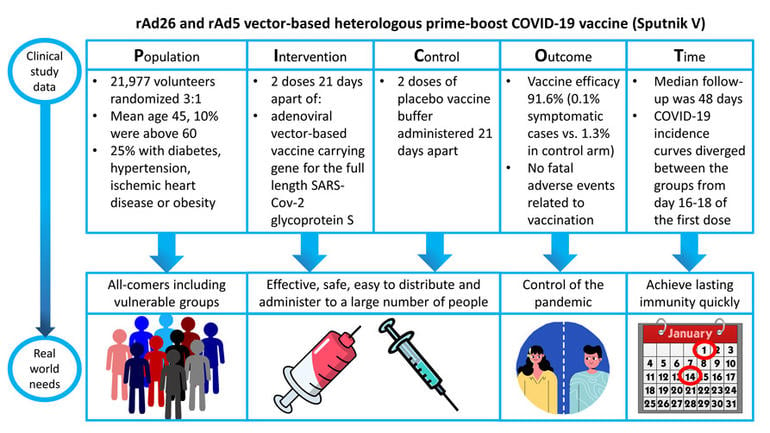
COVID-19: safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine (Sputnik V)
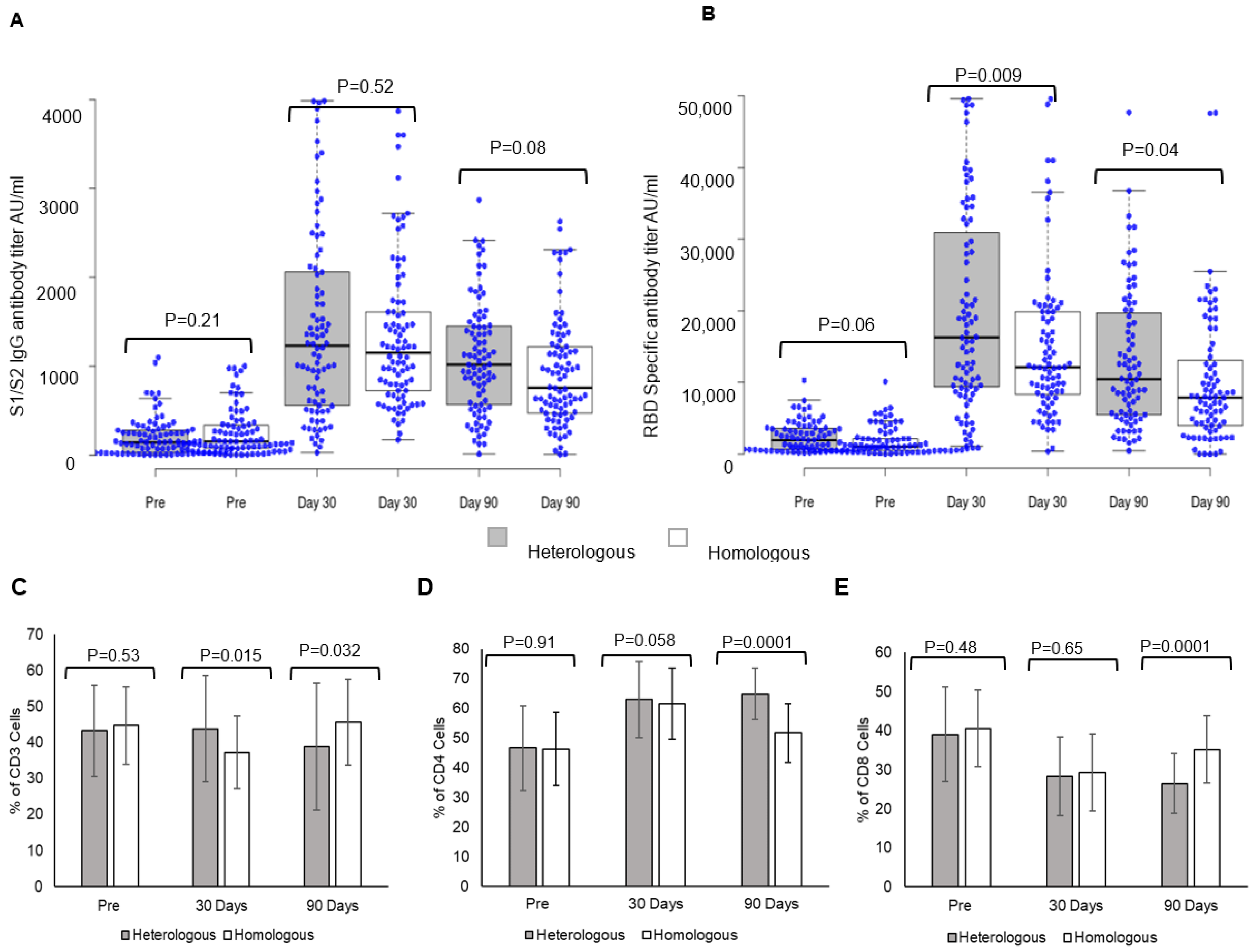
Vaccines | Free Full-Text | Heterologous Booster Dose with CORBEVAX following Primary Vaccination with COVISHIELD Enhances Protection against SARS-CoV-2

Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study - The Lancet Regional Health – Europe

Safety of heterologous primary and booster schedules with ChAdOx1-S and BNT162b2 or mRNA-1273 vaccines: nationwide cohort study | The BMJ
Strategies and safety considerations of booster vaccination in COVID-19 | Biomolecules and Biomedicine

Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study - The Lancet Respiratory Medicine

Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2 - The Lancet Infectious Diseases

Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study - The Lancet

Pivoting to protein: the immunogenicity and safety of protein-based NVX-CoV2373 as a heterologous booster for inactivated and viral vector COVID-19 vaccines: Expert Review of Vaccines: Vol 22, No 1
Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates